
Pioneer
Curative Therapies
Cureageous™
Therapies proven to be safe and effective in clinical trials are great first steps to lower the risks of relapse and improve lives. GJCF is proud to have played a leading role for this goal. And there is more to do. Beyond these breakthroughs, GJCF is now accelerating toward cures. It will take two bold steps to cure NMOSD, MOGAD and related autoimmune diseases once and for all: 1) Tolerization to reset the immune system so that it no longer targets self tissues; and 2) Regeneration to restore functions of tissues damaged by previous relapses. If we are not aiming for cures, we are not aiming high enough.
A decade ago, many believed that approved therapies would never be possible for a disease as rare as NMO. We believed otherwise. Guided by the revolutionary GJCF blueprint, there are now three therapies approved, and clinical trials are testing next-generation treatments for anti-AQP4, anti-MOG (MOGAD) and seronegative disease. Today, many believe that cures for NMOSD, MOGAD and other autoimmune diseases will never be possible. We believe otherwise.
Restore Immune Tolerance to End NMOSD/MOGAD Disease
The immune system protects the human body against many types of potential threats, including infection and cancer. It is also a key to tissue repair, healing and growth. To achieve these vital roles, the immune system must distinguish between healthy “self” tissue and unhealthy tissues, infection and cancer. This immune system superpower is called immune tolerance.
How Immune Tolerance Works
The immune system provides immunity to infection and cancer, and supports healthy tissue growth & repair. To do so it must constantly monitor all tissues in the body and respond in beneficial ways. A key to this role is the ability to tell the difference between healthy cells and molecules, and those that are abnormal. This capability is called immune tolerance. In some people, immune tolerance breaks down to certain proteins (such as AQP4 or MOG) over time. As a result, the immune system mistakes these proteins as abnormal, and targets inflammatory responses against them and the cells that express them (such as astrocytes and oligodendrocytes).
Loss of Immune Tolerance
For reasons that are not clearly understood, over time the immune system can mistake healthy “self” tissue as if it were unhealthy or foreign. This process results in the loss of immune tolerance. In NMOSD or MOGAD (MOG Antibody Disease), a loss of immune tolerance occurs to specific self proteins, AQP4 or MOG (Myelin oligodendrocyte glycoprotein). As a result, the immune system sees cells displaying such proteins as foreign, and injures them. Loss of immune tolerance is at the core of NMOSD and other autoimmune diseases. Solving immune tolerance in one autoimmune disease is likely to unlock to door to solving all autoimmune diseases!
Restoring Immune Tolerance
Immune tolerization represents the process of restoring immune tolerance. Tolerization re-educates immune system cells so they no longer see self tissues as threats, turning off autoimmune activity. The immune cells most important in tolerization are T and B cells along with other antigen presenting cells. If immune tolerance is restored immune suppressive therapies are not necessary, and the disease process is stopped. This is our goal. GJCF is working with academic, biotech and pharma partners on amazing new science that holds great promise to restore immune tolerance for cures.
Roadmap to Immune Tolerizing Cures.
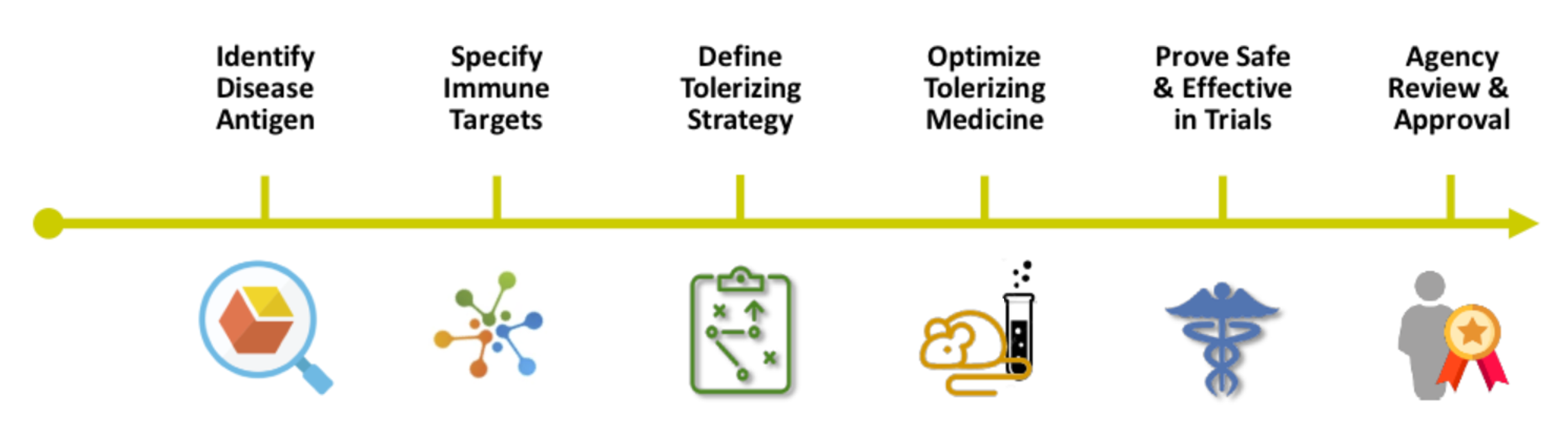
GJCF is Cureageous™
Curing diseases as complex as NMOSD or MOGAD is not easy. But if we do not aim for cures we are not aiming high enough. As with the first approved therapies, GJCF is leading the charge for cures – we are Cureageous™! Join our mission to cure NMOSD & MOGAD.
GJCF is iNMOtion to Cures
Bold new science, technology and clinical trials are already beginning to explore ways to restore immune tolerance in NMOSD or MOGAD. A few examples are shown here. Learn more about the GJCF mission for cures!

- Capsules delivering autoantigens to immune system
- Goal: help immune system to ignore AQP4 or MOG

- Inverse vaccines delivered through smart skin patch
- Goal: turn immune system down to AQP4 or MOG

- Nanoparticles delivering novel tolerizing medicines
- Goal: program immune tolerance to AQP4 or MOG

- Stem or autologous immune cell-based therapeutics
- Goal: re-educate or destroy autoreactive T / B cells

- Self red blood cells made to display AQP4 or MOG
- Goal: use a natural tolerizing method of the body

- AQP4 or MOG peptides designed as immune decoys
- Goal: turn off autoimmunity versus AQP4 or MOG
GJCF led in catalyzing the first-ever approved therapies to dramatically reduce risks of relapses for patients with NMOSD. These breakthroughs are saving lives-and they transformed neurology.
Now, GJCF is once again at the forefront of life-changing advances for those facing NMOSD. Discoveries driven by CIRCLES and other GJCF-funded research have enabled us to strive for cures.
This is a bold challenge-but if we are not aiming for cures-we are not aiming high enough.
Cures Take Courage. We Are Cureageous.
Applying its revolutionary blueprint, GJCF united experts, technologies and real-world experience to accelerate a biotech and pharma focus on NMOSD tolerization. Today, dozens of companies are developing tolerization therapies that may help cure NMOSD & end life-long immunosuppression.
Our road to tolerizing cures begins with NMOSD and MOGAD-but it could lead to cures for all autoimmune diseases. And technologies aimed at cures for type-1 diabetes, MS, lupus and other autoimmune diseases may help find cures for NMOSD and MOGAD. That is the power of rare.
Below are example biotech & pharma companies developing innovative tolerization technologies that are currently on the GJCF radar screen for their potential to help cure NMOSD & MOGAD.
|
Company |
Technology |
Mechanism |
Publication (sample) |
Stage |
|---|---|---|---|---|
|
Apoptotic DNA Immunotherapy |
Auto-Ag-targeted PCD of autoreactive APCs / Tc |
Pre-Clinical |
||
|
Auto-Ag Targeted TBPs to Oromucosa |
Tolerigenic bacterial particles (TBPs) target auto-Ag |
Pre-Clinical & IND |
||
|
Auto-Ag-Specific Tolerigenic NPs |
Auto-Ag targeting immune modulating NP plateform |
Pre-Clinical |
||
|
Auto-Ag-Specific Hepato-RBC Vax |
Liver-RBC reticuloendothelial inverse tolerigenic vaccine |
Phase I/II & Pre-Clinical |
||
|
Engineered Auto-Ag Decoy Apitopes |
Tc anergizing & Tr1 / FoxP3+ Treg inducing apitopes |
Phase IIa |
||
|
Autologous DC Tolerization Ex Vivo |
Auto-Ag peptide-loading induces tolerigenic DC |
Phase Ib |
||
|
Auto-Ag Specific Ψ mRNA Vax |
Auto-Ag presentation w/o 2nd signal induces tolerization |
Pre-Clinical |
||
|
[ Novartis ] |
Auto-Ag Peptide Coupled RBC |
Exploit tolerigenic systems in MHC-pathway RBC tolerance |
TBD |
Phase I/II |
|
Affinity-attenuated IL-2 Variant |
Modulatory costimulatory ligands targeting ARTc |
Pre-Clinical |
||
|
PGLA-Auto-Ag Nanoparticle |
Target hepatic APC for tolerigenic reprogramming |
Phase II |
||
|
Auto-Ag Specific LN / Oral Vaccines |
Intralymphatic vax targeting Auto-Ag / APC / Tc Crosstalk |
Phase I / II |
||
|
[ Amgen ] |
Auto-Ag-Targeted Nanodisc Vaccine |
High-efficiency targeting of auto-Ag to APC for tolerization |
Phase I & Pre-Clinical |
|
|
Autologous Dc Tolerization Ex Vivo |
Tolerigenic DC reprogramming for Auto-Ag specific tolerance |
Phase IIa & Pre-Clinical |
||
|
High-affinity tolerigenic Abs |
Chimeric IgE/IgG4 Ab confer tolerigenesis to specific Ag |
Pre-Clinical |
||
|
Thio-Imotope Modulating Peptide |
Imotope-specific CD4 Tc target Auto-Ag reactive APCs |
Phase II |
||
|
Autologous & Allo CARTc Therapy |
CARTc targeting of CD19+ Bc using chimeric-Ag receptors |
Phase I |
||
|
CD34+ Donor HSC |
CD34+ Allo-HSC chimerism to induce tolerigenic paradigm |
Phase III |
||
|
Novel plasmid DNA targeted immunoTx |
Targeted auto-Ag expression for tolerigenesis induction |
Phase I |
||
|
Ag/APC-Specific Vaccibody Platform |
Ab-Targeting of Auto-Ag/BcR to APCs for Tolerization |
Phase I & Pre-Clinical |
||
|
[ Genentech ] |
Navacims (Auto-Ag MHC Nanoparticle) |
Navacim-generated auto-Ag specific Treg induction |
Pre-Clinical |
|
|
Small molecule inhibitor program |
Targeted inhibition of key inflammation pathways |
Phase I, II and III |
||
|
Modify Autologous RBC with Target Auto-Ag |
Auto-Ag decorated RBCs for tolerigenic induction |
Pre-Clinical |
||
|
Auto-Ag specific ImmTOR NPs |
ImmTOR nanoparticles target APCs induces Ag-specific Treg |
Phase I & Pre-Clinical |
||
|
Modify Autologous RBC with Auto-Ag |
Multi-modal tolerigenesis via MHC-pathway RBC tolerance |
Pre-Clinical |
||
|
Facilitated FCR001 Allo-HSC Transplant |
CD34+ / ab Tc-facilitated allogenic HSC transplant |
Phase I & II |
||
|
Auto-Ag specific tolerogen peptides |
Auto-Ag targeted tolerogens induce Treg tolerization |
TBD |
Pre-Clinical |
|
|
[ Eli Lily ] |
NP-Targeted Auto-Ag Therapy |
Liver sinusoidal endothelial cell targeted auto-Ag nanoparticle |
Phase I & Pre-Clinical |
|
|
Cures Take Courage . We Are Cureageous. TM |
||||
Note: information subject to change; see specific company website. Bracketed font denotes the parental or partner company.
Glossary
Ab, antibody; Ag, antigen; Allo-HSC, allogenic (donated) human stem cell; APC, antigen presenting cell; ARBc, autoreactive B cell; ARTc, autoreactive T cell; Auto-Ag, auto-antigen; Bc, B cell; CAR-Tc, chimeric antigen receptor T cell; CAAR-Tc, chimeric auto-antigen receptor T cell; CD4 Tc, CD4+ T cell; CD8 Tc, CD8+ T cell; CD19+, CD19 marker of B cells; Dc, dendritic cell; Dz, disease; DNA, deoxyribonucleic acid; EAE, experimental autoimmune encephalomyelitis; HLA, human leukocyte antigen; HSC, human stem cell; IgE, immunoglobulin E class; IgG, immunoglobulin G class; IL-2, interleukin-2; LN, lymph node; mRNA, messenger ribonucleic acid; MHC, major histocompatibility complex; MS, multiple sclerosis; NPs, nanoparticles; PCD, programmed cell death (apoptosis); RBC, red blood cell; RNA, ribonucleic acid; Tc, T cell; Tr1, Tr1 regulatory T cell; Treg, regulatory T cell; Tx, therapy; Vax, vaccine
Next: PROMOTE | Advocacy for All
Enhance Patient Experience Through Education & Advocacy
Patients, caregivers and loved ones are best served with credible information and healthcare access. GJCF promotes wellness via education and advocacy.

